- Lewis Electron Dot Structure
- Lewis Electron Dot Structure Calculator Form
- Lewis Electron Dot Structure Calculator Equation
- Lewis Electron Dot Structure Calculator
- Lewis Dot Diagram Calc
- Draw the Lewis electron dot structures for the following compound. Calculate the formal charge on each atom in wach structure and if theres is more than one possible structure for the compound use formal charge to indicate the more likely structure. Label all bonds as being polar or nonpolar.
- A step-by-step explanation of how to draw the MgF2 Lewis Dot Structure.For MgF2 we have an ionic compound and we need to take that into account when we draw.
Learning Objectives
To generate the Lewis dot structure, you have to follow the given steps: Find the total count of valence electrons to molecules. In this step, add the total count of valence electrons from all the atoms in a bit. Find the required count of electrons needed to make the atoms complete. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Get the free 'Lewis structure' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha.
- State the octet rule.
- Define ionic bond.
- Draw Lewis structures for ionic compounds.
In Section 4.7 we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anions. The astute reader may have noticed something: many of the ions that form have eight electrons in their valence shell. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons in their original valence shell; the lower shell, now the valence shell, has eight electrons in it, so the atom becomes positively charged. For whatever reason, having eight electrons in a valence shell is a particularly energetically stable arrangement of electrons. The octet rule explains the favorable trend of atoms having eight electrons in their valence shell. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make.
It is not impossible to violate the octet rule. Consider sodium: in its elemental form, it has one valence electron and is stable. It is rather reactive, however, and does not require a lot of energy to remove that electron to make the Na+ ion. We could remove another electron by adding even more energy to the ion, to make the Na2+ ion. However, that requires much more energy than is normally available in chemical reactions, so sodium stops at a 1+ charge after losing a single electron. It turns out that the Na+ ion has a complete octet in its new valence shell, the n = 2 shell, which satisfies the octet rule. The octet rule is a result of trends in energies and is useful in explaining why atoms form the ions that they do.
Now consider an Na atom in the presence of a Cl atom. The two atoms have these Lewis electron dot diagrams and electron configurations:
[mathbf{Na, cdot }; ; ; ; ; ; ; ; ; ; mathbf{cdot }mathbf{ddot{underset{.: .}Cl}}mathbf{: :}]
[left [ Ne right ]3s^{1}; ; ; ; left [ Ne right ]3s^{2}3p^{5}]
For the Na atom to obtain an octet, it must lose an electron; for the Cl atom to gain an octet, it must gain an electron. An electron transfers from the Na atom to the Cl atom:
[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.: .}Cl}}mathbf{: :}]
resulting in two ions—the Na+ ion and the Cl− ion:
[mathbf{Na}^{+}; ; ; ; ; ; ; ; mathbf{:}mathbf{ddot{underset{.: .}Cl}}mathbf{: :}^{-}]
[left [ Ne right ]; ; ; ; ; left [ Ne right ]3s^{2}3p^{6}]
Both species now have complete octets, and the electron shells are energetically stable. From basic physics, we know that opposite charges attract. This is what happens to the Na+ and Cl− ions:
[mathbf{Na}^{+}; + ; mathbf{:}mathbf{ddot{underset{.: .}Cl}}mathbf{: :}^{-}rightarrow Na^{+}Cl^{-}; ; or; ; NaCl]
where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges explicitly. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another.
In electron transfer, the number of electrons lost must equal the number of electrons gained. We saw this in the formation of NaCl. A similar process occurs between Mg atoms and O atoms, except in this case two electrons are transferred:
The two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond:
[mathbf{Mg,}^{2+}; + ; left[mathbf{:}mathbf{ddot{underset{.: .}O}}mathbf{: :}right]^{2-}; ; ; ; ; Mg^{2+}O^{2-}; or; MgO]
Remember, in the final formula for the ionic compound, we do not write the charges on the ions.
What about when an Na atom interacts with an O atom? The O atom needs two electrons to complete its valence octet, but the Na atom supplies only one electron:
[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.}O}}mathbf{: :}]

The O atom still does not have an octet of electrons. What we need is a second Na atom to donate a second electron to the O atom:
These three ions attract each other to give an overall neutral-charged ionic compound, which we write as Na2O. The need for the number of electrons lost being equal to the number of electrons gained explains why ionic compounds have the ratio of cations to anions that they do. This is required by the law of conservation of matter as well.
Lewis Electron Dot Structure
Example (PageIndex{1}): Synthesis of calcium chloride from Elements
With arrows, illustrate the transfer of electrons to form calcium chloride from (Ca) atoms and (Cl) atoms.
Solution
A (Ca) atom has two valence electrons, while a (Cl) atom has seven electrons. A (Cl) atom needs only one more to complete its octet, while (Ca) atoms have two electrons to lose. Thus we need two (Cl) atoms to accept the two electrons from one (Ca) atom. The transfer process looks as follows:
The oppositely charged ions attract each other to make CaCl2.
Exercise (PageIndex{1})
With arrows, illustrate the transfer of electrons to form potassium sulfide from (K) atoms and (S) atoms.
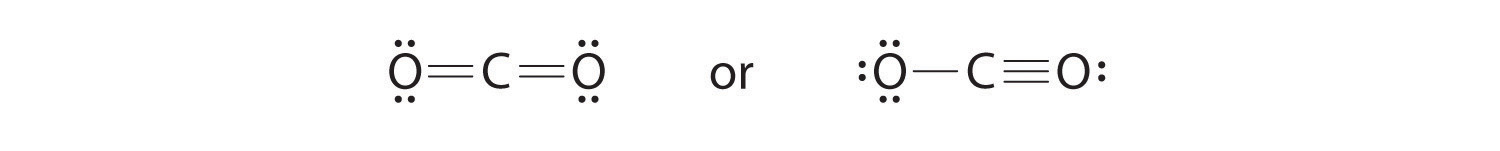
- Answer
Summary
- The tendency to form species that have eight electrons in the valence shell is called the octet rule.
- The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.
- The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions.
Contributions & Attributions

This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
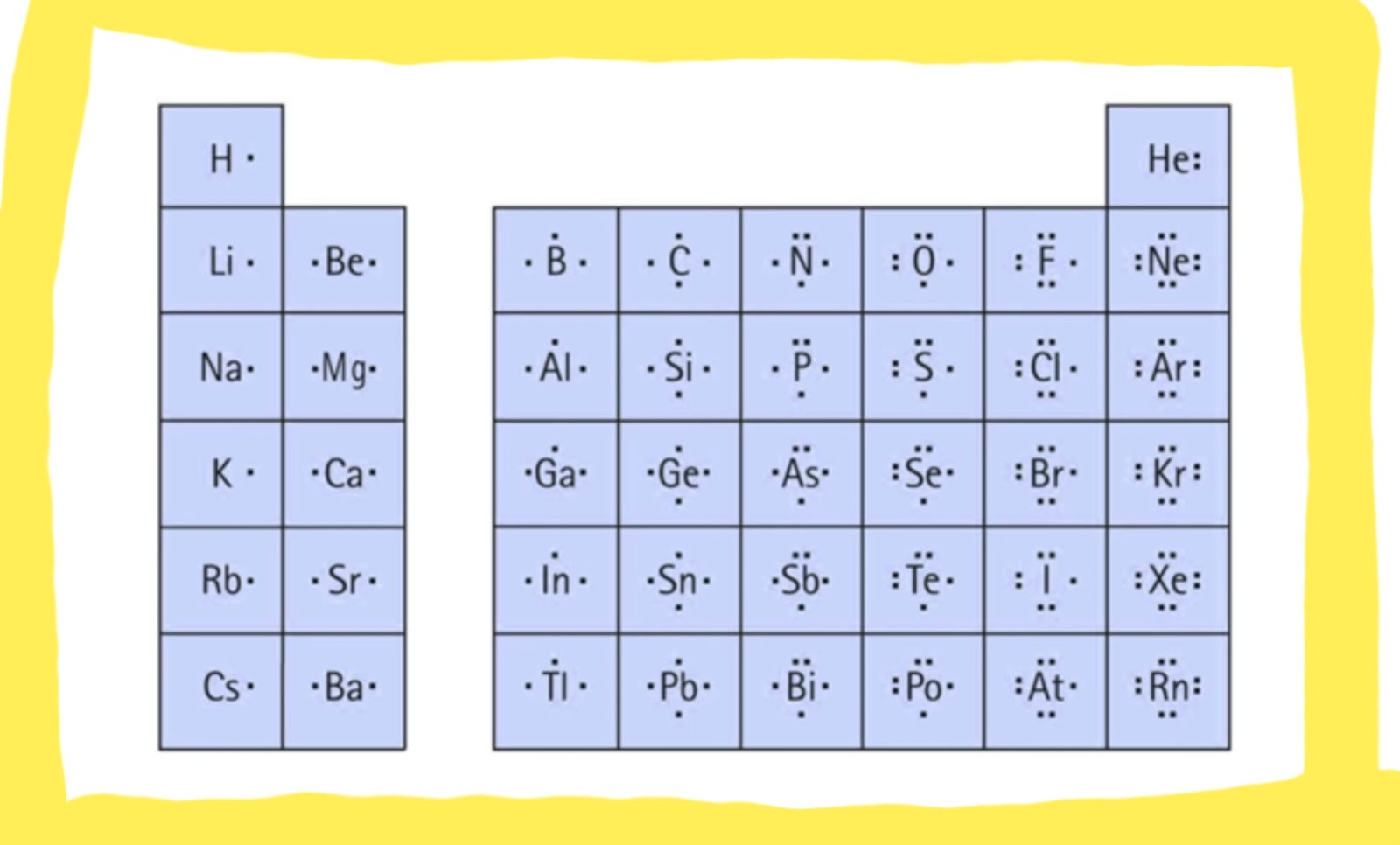
Using Lewis Dot Symbols to Describe Covalent Bonding
This sharing of electrons allowing atoms to 'stick' together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.1 nm, or if you prefer 100 pm, at which the attractive forces significantly outweigh the repulsive forces and a bond will be formed if both atoms can achieve a completen s2np6 configuration. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl2, they can each complete their valence shell:
Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond.
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols:
The structure on the right is the Lewis electron structure, or Lewis structure, for H2O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples:
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
- Arrange the atoms to show specific connections. When there is a central atom, it is usually the least electronegative element in the compound. Chemists usually list this central atom first in the chemical formula (as in CCl4 and CO32−, which both have C as the central atom), which is another clue to the compound's structure. Hydrogen and the halogens are almost always connected to only one other atom, so they are usually terminal rather than central.
Note:
The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal.
- Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall from Chapter 2 that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. For CO32−, for example, we add two electrons to the total because of the −2 charge.
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In H2O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
- Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). These electrons will usually be lone pairs.
- If any electrons are left over, place them on the central atom. Some atoms are able to accommodate more than eight electrons.
- If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. This will not change the number of electrons on the terminal atoms.
Now let's apply this procedure to some particular compounds, beginning with one we have already discussed.
H2O
1. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons
3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over.
4. Each H atom has a full valence shell of 2 electrons.
5. Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:
This is the Lewis structure we drew earlier. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6.
1. With only two atoms in the molecule, there is no central atom.
2. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons between O and Cl gives O:Cl, with 12 electrons left over.
4. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure:

where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges explicitly. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another.
In electron transfer, the number of electrons lost must equal the number of electrons gained. We saw this in the formation of NaCl. A similar process occurs between Mg atoms and O atoms, except in this case two electrons are transferred:
The two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond:
[mathbf{Mg,}^{2+}; + ; left[mathbf{:}mathbf{ddot{underset{.: .}O}}mathbf{: :}right]^{2-}; ; ; ; ; Mg^{2+}O^{2-}; or; MgO]
Remember, in the final formula for the ionic compound, we do not write the charges on the ions.
What about when an Na atom interacts with an O atom? The O atom needs two electrons to complete its valence octet, but the Na atom supplies only one electron:
[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.}O}}mathbf{: :}]
The O atom still does not have an octet of electrons. What we need is a second Na atom to donate a second electron to the O atom:
These three ions attract each other to give an overall neutral-charged ionic compound, which we write as Na2O. The need for the number of electrons lost being equal to the number of electrons gained explains why ionic compounds have the ratio of cations to anions that they do. This is required by the law of conservation of matter as well.
Lewis Electron Dot Structure
Example (PageIndex{1}): Synthesis of calcium chloride from Elements
With arrows, illustrate the transfer of electrons to form calcium chloride from (Ca) atoms and (Cl) atoms.
Solution
A (Ca) atom has two valence electrons, while a (Cl) atom has seven electrons. A (Cl) atom needs only one more to complete its octet, while (Ca) atoms have two electrons to lose. Thus we need two (Cl) atoms to accept the two electrons from one (Ca) atom. The transfer process looks as follows:
The oppositely charged ions attract each other to make CaCl2.
Exercise (PageIndex{1})
With arrows, illustrate the transfer of electrons to form potassium sulfide from (K) atoms and (S) atoms.
- Answer
Summary
- The tendency to form species that have eight electrons in the valence shell is called the octet rule.
- The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.
- The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
Using Lewis Dot Symbols to Describe Covalent Bonding
This sharing of electrons allowing atoms to 'stick' together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.1 nm, or if you prefer 100 pm, at which the attractive forces significantly outweigh the repulsive forces and a bond will be formed if both atoms can achieve a completen s2np6 configuration. It is this behavior that Lewis captured in his octet rule. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl2, they can each complete their valence shell:
Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair ; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond.
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols:
The structure on the right is the Lewis electron structure, or Lewis structure, for H2O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples:
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
- Arrange the atoms to show specific connections. When there is a central atom, it is usually the least electronegative element in the compound. Chemists usually list this central atom first in the chemical formula (as in CCl4 and CO32−, which both have C as the central atom), which is another clue to the compound's structure. Hydrogen and the halogens are almost always connected to only one other atom, so they are usually terminal rather than central.
Note:
The central atom is usually the least electronegative element in the molecule or ion; hydrogen and the halogens are usually terminal.
- Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall from Chapter 2 that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. For CO32−, for example, we add two electrons to the total because of the −2 charge.
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. In H2O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
- Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). These electrons will usually be lone pairs.
- If any electrons are left over, place them on the central atom. Some atoms are able to accommodate more than eight electrons.
- If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the central atom to achieve an octet. This will not change the number of electrons on the terminal atoms.
Now let's apply this procedure to some particular compounds, beginning with one we have already discussed.
H2O
1. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH.
2. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons
3. Placing one bonding pair of electrons between the O atom and each H atom gives H:O:H, with 4 electrons left over.
4. Each H atom has a full valence shell of 2 electrons.
5. Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:
This is the Lewis structure we drew earlier. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6.
1. With only two atoms in the molecule, there is no central atom.
2. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Placing a bonding pair of electrons between O and Cl gives O:Cl, with 12 electrons left over.
4. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure:
Each atom now has an octet of electrons, so steps 5 and 6 are not needed. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. OCl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
1. Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. One possible arrangement is as follows:
2. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons.
3. Placing a bonding pair of electrons between each pair of bonded atoms gives the following:
Six electrons are used, and 6 are left over.
4. Adding all 6 remaining electrons to oxygen (as three lone pairs) gives the following:
Although oxygen now has an octet and each hydrogen has 2 electrons, carbon has only 6 electrons.
5. There are no electrons left to place on the central atom.
6. To give carbon an octet of electrons, we use one of the lone pairs of electrons on oxygen to form a carbon–oxygen double bond:
Both the oxygen and the carbon now have an octet of electrons, so this is an acceptable Lewis electron structure. The O has two bonding pairs and two lone pairs, and C has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid.
An alternative structure can be drawn with one H bonded to O. Formal charges, discussed later in this section, suggest that such a structure is less stable than that shown previously.
Example
Write the Lewis electron structure for each species.
- NCl3
- S22−
- NOCl
Given: chemical species
Asked for: Lewis electron structures
Strategy:
Use the six-step procedure to write the Lewis electron structure for each species.
Show AnswerNitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States.
In a diatomic molecule or ion, we do not need to worry about a central atom. Each sulfur atom (group 16) contains 6 valence electrons, and we need to add 2 electrons for the −2 charge, giving a total of 14 valence electrons. Using 2 electrons for the S–S bond, we arrange the remaining 12 electrons as three lone pairs on each sulfur, giving each S atom an octet of electrons:
Because nitrogen is less electronegative than oxygen or chlorine, it is the central atom. The N atom (group 15) has 5 valence electrons, the O atom (group 16) has 6 valence electrons, and the Cl atom (group 17) has 7 valence electrons, giving a total of 18 valence electrons. Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following:
Adding three lone pairs each to oxygen and to chlorine uses 12 more electrons, leaving 2 electrons to place as a lone pair on nitrogen:
Because this Lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to form a bonding pair. We could use a lone pair on either O or Cl. Because we have seen many structures in which O forms a double bond but none with a double bond to Cl, it is reasonable to select a lone pair from O to give the following:
All atoms now have octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas.
Example
Write Lewis electron structures for CO2 and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule, as we saw for CH2O, but not every Lewis structure may be equally reasonable. In these situations, we can choose the most stable Lewis structure by considering the formal charge on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. A formal charge does not represent a true charge on an atom in a covalent bond but is simply used to predict the most likely structure when a compound has more than one valid Lewis structure.
To calculate formal charges, we assign electrons in the molecule to individual atoms according to these rules:
- Nonbonding electrons are assigned to the atom on which they are located.
- Bonding electrons are divided equally between the bonded atoms.
For each atom, we then compute a formal charge:
formal charge = valence e−−(free atom)(non−bonding e− + bonding e−/2)
To illustrate this method, let's calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows:
A neutral nitrogen atom has five valence electrons (it is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [2 nonbonding e− + (6 bonding e−/2)]. Substituting into the below equation, we obtain:
formal charge(N)= 5 valence e–−(2non−bonding e− + 6 bonding e−/2)=0
A neutral hydrogen atom has one valence electron. Each hydrogen atom in the molecule shares one pair of bonding electrons and is therefore assigned one electron [0 nonbonding e− + (2 bonding e−/2)]. Using the below equation to calculate the formal charge on hydrogen, we obtain:
formal charge(H)= 1 valence e–−(0 non−bonding e− + 2 bonding e−/2)=0
The hydrogen atoms in ammonia have the same number of electrons as neutral hydrogen atoms, and so their formal charge is also zero. Adding together the formal charges should give us the overall charge on the molecule or ion. In this example, the nitrogen and each hydrogen has a formal charge of zero. When summed the overall charge is zero, which is consistent with the overall charge on the NH3 molecule.
Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. In cases where there are positive or negative formal charges on various atoms, stable structures generally have negative formal charges on the more electronegative atoms and positive formal charges on the less electronegative atoms. The next example further demonstrates how to calculate formal charges.
Example
Calculate the formal charges on each atom in the NH4+ ion.
Given: chemical species
Asked for: formal charges
Strategy:
Identify the number of valence electrons in each atom in the NH4+ ion. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use the given formula to calculate the formal charge on each atom.
Show AnswerThe Lewis electron structure for the NH4+ ion is as follows:
The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using the formula, the formal charge on the nitrogen atom is
formal charge(N)=5−(0+8/2)=1
Each hydrogen atom in has one bonding pair. The formal charge on each hydrogen atom is therefore
formal charge(H)=1−(0+2/2)=0
The formal charges on the atoms in the NH4+ ion are thus
Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +1.
Example
Write the formal charges on all atoms in BH4−.
Show AnswerIf an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero.
Using Formal Charges to Distinguish between Lewis Structures
As an example of how formal charges can be used to determine the most stable Lewis structure for a substance, we can compare two possible structures for CO2. Both structures conform to the rules for Lewis electron structures.
1. C is less electronegative than O, so it is the central atom.
2. C has 4 valence electrons and each O has 6 valence electrons, for a total of 16 valence electrons.
3. Placing one electron pair between the C and each O gives O–C–O, with 12 electrons left over.
4. Dividing the remaining electrons between the O atoms gives three lone pairs on each atom:
This structure has an octet of electrons around each O atom but only 4 electrons around the C atom.
5. No electrons are left for the central atom.
6. To give the carbon atom an octet of electrons, we can convert two of the lone pairs on the oxygen atoms to bonding electron pairs. There are, however, two ways to do this. We can either take one electron pair from each oxygen to form a symmetrical structure or take both electron pairs from a single oxygen atom to give an asymmetrical structure:
Both Lewis electron structures give all three atoms an octet. How do we decide between these two possibilities? The formal charges for the two Lewis electron structures of CO2 are as follows:
Both Lewis structures have a net formal charge of zero, but the structure on the right has a +1 charge on the more electronegative atom (O). Thus the symmetrical Lewis structure on the left is predicted to be more stable, and it is, in fact, the structure observed experimentally. Remember, though, that formal charges do not represent the actual charges on atoms in a molecule or ion. They are used simply as a bookkeeping method for predicting the most stable Lewis structure for a compound.
Note:
The Lewis structure with the set of formal charges closest to zero is usually the most stable.
Examples
The thiocyanate ion (SCN−), which is used in printing and as a corrosion inhibitor against acidic gases, has at least two possible Lewis electron structures. Draw two possible structures, assign formal charges on all atoms in both, and decide which is the preferred arrangement of electrons.
Given: chemical species
Asked for: Lewis electron structures, formal charges, and preferred arrangement
Strategy:
A Use the step-by-step procedure to write two plausible Lewis electron structures for SCN−. Multicharts on mac os.
Lewis Electron Dot Structure Calculator Form
B Calculate the formal charge on each atom using formal charge = valence e−−(free atom)(non−bonding e− + bonding e−/2)
Lewis Electron Dot Structure Calculator Equation
C Predict which structure is preferred based on the formal charge on each atom and its electronegativity relative to the other atoms present.
Show AnswerA Possible Lewis structures for the SCN− ion are as follows:
B We must calculate the formal charges on each atom to identify the more stable structure. If we begin with carbon, we notice that the carbon atom in each of these structures shares four bonding pairs, the number of bonds typical for carbon, so it has a formal charge of zero. Continuing with sulfur, we observe that in (a) the sulfur atom shares one bonding pair and has three lone pairs and has a total of six valence electrons. The formal charge on the sulfur atom is therefore 6−(6+2/2)=−1 and 5−(4+4/2)=−1. In (c), nitrogen has a formal charge of −2.
C Which structure is preferred? Structure (b) is preferred because the negative charge is on the more electronegative atom (N), and it has lower formal charges on each atom as compared to structure (c): 0, −1 versus +1, −2.
Example
Lewis Electron Dot Structure Calculator
Salts containing the fulminate ion (CNO−) are used in explosive detonators. Draw three Lewis electron structures for CNO− and use formal charges to predict which is more stable. (Note: N is the central atom.)
Show AnswerThe second structure is predicted to be more stable.
Lewis Dot Diagram Calc
Contributors
- Anonymous
